AI-based Medical Devices and the FDA’s Predetermined Change Control Plans (PCCPs)
PatentNext Takeaway: This post highlights the FDA’s increasing regulatory efforts for artificial intelligence (AI) and machine learning (ML)-enabled medical devices (MLMDs), with a focus on managing device AI/ML updates through Predetermined Change Control Plans (PCCPs). The FDA emphasizes five guiding principles for PCCPs to ensure safety, risk management, and transparency for MLMDs throughout their lifecycle. The post also notes a significant rise in AI-based medical device FDA submissions and related patent filings, particularly since 2016, indicating growing interest in this technology.
****
Introduction
The Food and Drug Administration (FDA) continues to engage in regulatory efforts for Artificial Intelligence (AI) and machine learning (ML) based software devices, which the FDA refers to as artificial intelligence/machine learning-enabled medical devices (MLMD). This article serves as an update to those efforts, as previously discussed on PatentNext. See PatentNext: The Intersection of Artificial Intelligence (AI), Life Sciences, Healthcare, and Intellectual Property (IP).
AI-based Medical Device FDA Filings
Innovators of AI-based medical device inventions should familiarize themselves with the regulatory landscape, including the FDA’s position on Artificial Intelligence (AI) and machine learning (ML) based software devices, including MLMDs.
The FDA has identified AI/ML as an important technology that it will monitor and regulate, stating that “AI/ML technologies have the potential to transform health care by deriving new and important insights from the vast amount of data generated during the delivery of health care every day,” and that “[t]he FDA may also review and clear modifications to medical devices, including software as a medical device, depending on the significance or risk posed to patients of that modification.” Artificial Intelligence and Machine Learning in Software as a Medical Device” (FDA.gov).
The FDA maintains a list of AI/ML-enabled Medical devices submitted to the FDA. The list includes devices submitted and authorized via 510(k) clearance, granted De Novo request, or an approved Premarket Approval Application (PMA).
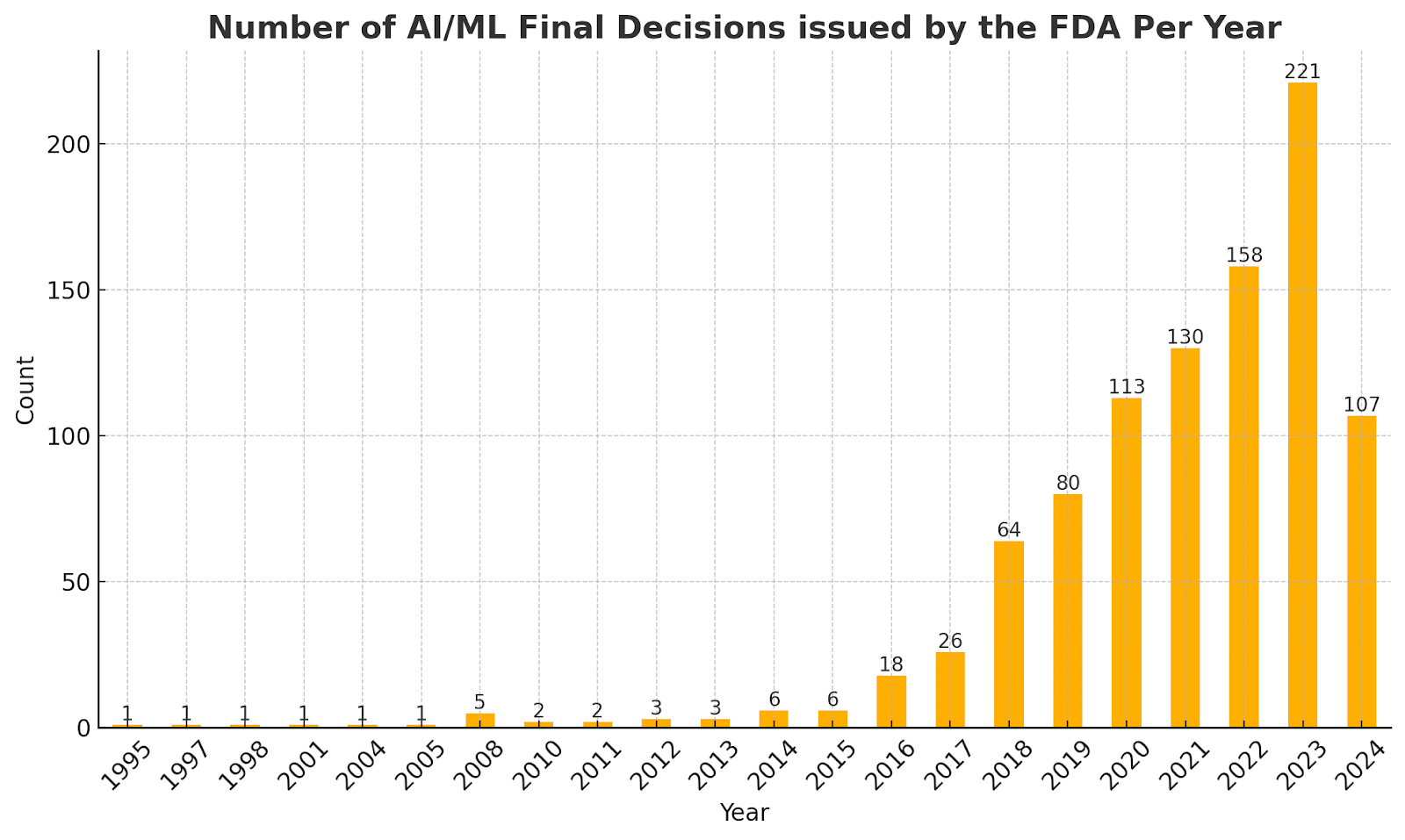
The chart below summarizes the number of AI/ML submissions made to the FDA through the years.
Chart 1: Number of AI/ML Final Decisions by the FDA Per Year
As shown in the above Chart 1, AI/ML decisions started increasing exponentially around the 2016 timeframe. This correlates with AI-medical device patent filings, as shown below in this article.
As shown in the below Chart 2, by far, the Radiology FDA panel received the most submissions (723 submissions) compared to any other group. The Cardiovascular FDA panel received the second most submissions (98 submissions). The remaining panels each received 35 or fewer submissions.
Chart 2: AI/ML Submissions by FDA Panel Type
The FDA’s Predetermined Change Control Plans (PCCPs) for updating AI/ML-enabled medical devices
Even after being initially approved by the FDA, an AI/ML-enabled medical device may be subject to additional approval when its AI model is updated. This is because an update to a medical device’s underlying AI model may cause the AI model, and thus the AI/ML-enabled medical device, to operate differently from when it was originally approved by the FDA.
The FDA acknowledges that traditional medical device regulation wasn’t designed to address technology that can update an already approved device, e.g., “The FDA’s traditional paradigm of medical device regulation was not designed for adaptive artificial intelligence and machine learning technologies. Many changes to artificial intelligence and machine learning-driven devices may need a premarket review.” “Artificial Intelligence and Machine Learning in Software as a Medical Device” (FDA.gov).
Further, the FDA acknowledges that changes to an AI model deployed on an approved AI medical device can be significant, e.g., “certain changes to [machine learning-enabled medical devices] MLMDs, such as changes to a model or algorithm, may be substantive or significant. For this reason, they can require regulatory oversight, such as additional premarket review. Such regulatory expectations may not always coincide with the rapid pace of MLMD development.” Predetermined Change Control Plans for Machine Learning-Enabled Medical Devices: Guiding Principles (FDA.gov).
In order to address this issue, the FDA has released a set of five (5) “guiding principles” to manage and monitor MLMDs, having deployed AI models for performance and re-training risks. Id. The FDA looks to these five guiding principles to develop or review Predetermined Change Control Plans (PCCPs).
The 5 Guiding Principles for developing a PCCP are summarized below.
1. Focused and Bounded: A PCCP should describe specific changes that a manufacturer intends to implement. Such changes should be limited to modifications within the intended use or intended purpose of the original MLMD.
2. Risk-based: A PCCP should be driven by a risk-based approach throughout the MLMD’s product lifecycle to ensure that individual and cumulative changes remain appropriate over time for the MLMD and its use environment.
3. Evidence-Based: A PCCP should involve collection/monitoring evidence to ensure the ongoing safety and effectiveness of the MLMD, demonstrate that the benefits outweigh the associated risks, and establish that the risks are adequately managed and controlled.
4. Transparent: A PCCP should provide transparency to users and/or stakeholders of the MLMD by, e.g., characterization of data used in the development and modifications of a model deployed on the MLMD, comprehensive testing for planned changes, characterization of the MLMD before and after implementation of changes, and monitoring, detection, and response to deviations in MLMD performance.
5. Total Product Lifecycle (TPLC) Perspective: A PCCP should consider the lifecycle of the product by employing risk management practices during the lifecycle of the MLMD and by using and supporting existing regulatory, quality, and risk management measures throughout the TPLC to ensure device safety by monitoring, reporting and responding to safety concerns.
Currently, the 5 Guiding Principles do not replace existing FDA regulatory procedures or constitute approval of any updated AI/ML-enabled Medical device. Instead, the 5 Guiding Principles are to “facilitate and foster ongoing engagement and collaboration among stakeholders on the PCCP concept for MLMD” and to “lay a foundation for PCCPs and encourage international harmonization.” Id.
AI-based Medical Device Patent Filings
The number of AI-based Medical Device Patent filings follows a similar trend compared to the number of AI/ML submissions made to the FDA, as shown above in Chart 1.
For example, the below chart shows AI and Life Science patent filings by Technology (“Tech”) Center over time from 2000 to 2024, where a spike exists in filing activity post-2016 (note that the right-most side of the graph slopes downward because of the 18-month “Publication Delay,” during which information for newer patent application filings, is not yet publicly available. See 37 CFR § 1.211).
The above chart is provided courtesy of Juristat. The chart and information were obtained by searching for “software AND medical AND device AND (‘artificial intelligence’ OR ‘machine learning’)” and using the fields Title, Abstract, Description, and Claims in the Juristat app.
After the spike in 2016, the above chart shows continued activity up to the present day. The above chart organizes patent application filings by USPTO Tech Centers. As shown, most AI-based medical device patent filings fall within Tech Center 2600 (“Communications”), having received, for example, about 2500 filings in the year 2022. This tech center includes art units that focus on computer graphic processing (art unit 2615) and image analysis (art unit 2660), which are important to radiotherapy and radiology inventions. These technical areas can relate to radiology, which uses imaging technology, and thus correlates to Chart 2 above regarding FDA filings in the radiology field.
Given the increased interest in AI and medical device technology and the increase in FDA submissions, as discussed above, we can expect further filings in this space in the coming years.
****
Subscribe to get updates to this post or to receive future posts from PatentNext. Start a discussion or reach out to the author, Ryan Phelan, at rphelan@marshallip.com (Tel: 312-474-6607). Connect with or follow Ryan on LinkedIn.







